Lower GI – FIT Testing

Thank you for your support in the increased use of the FIT tests when referring patients with a suspected colorectal cancer. We have seen a huge increase in the number of referrals arriving with a FIT result which ensures our patients go to the right place first time.
We continue to encourage the use of the FIT testing as it provides a high level of assurance to patients with negative results (99.7%). It is more accurate than a colonoscopy which means intrusive and unnecessary investigation for patients can be avoided in most cases.
"FIT will support patients to have the right referral, investigation and management at the right time and according to their clinical risk of colorectal cancer”
Dr Debbie Harvey, GP Lead, Cheshire & Mersey Cancer Alliance
- Update on FIT – May 2024
Change to process for GPs - ordering FIT kit
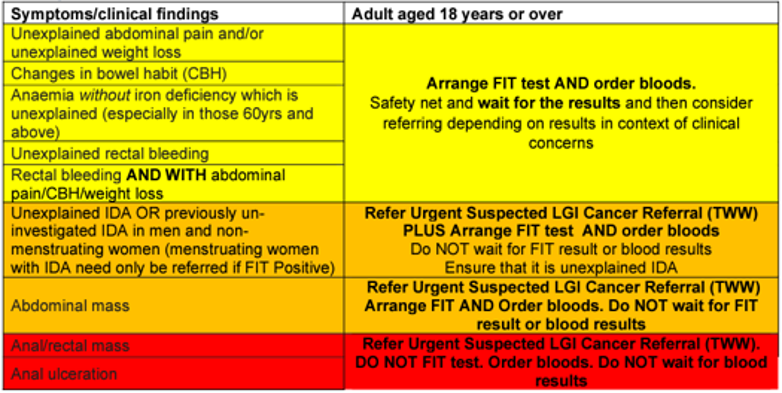
In April 2023, the process for lower GI referrals changed and GPs were asked to complete FIT before sending in referrals, except in certain specific cases.
At that time, a decision was made in STHK to retain the legacy kit-ordering processes which had been in place since the launch of FIT in 2020. GPs ordered FIT via ICE and kits were dispatched to patients from the STHK pathology team.
Elsewhere in the Cheshire and Mersey catchment, a different approach was piloted, and after an initial stock was provided, GPs ordered FIT kit in the same way that orders for urine bottle and similar test materials are processed.
This FIT kit approach has proved to be successful and simplifies matters throughout the process.
STHK is now part of MWL, and it is time for us to fall in line with other providers across Cheshire and Mersey.
An initial stock of FIT kit will be delivered to each practice between Tuesday May 7th and Thursday May 9th. The number of kits in this delivery will have been agreed with your local Place team.
It will be addressed to a named person – please ensure that the stock is held ready for the launch of the revised process on Monday May 13th, and NOT issued beforehand.
Thereafter, practices should re-order stock as demand indicates, via the existing processes for pathology consumables.
If you have any queries or concerns, please review the FAQs below, and if appropriate, via your local clinical lead for FIT.
- Frequently Asked Questions (FAQs)
What changes on 13th May?
The revised system for managing FIT kit begins.
By then, practices will have received an initial stock of FIT kit and guidance on the new process.
How will initial supplies be provided to each practice?
An initial stock will be delivered by post on 8-10 May
What safety netting processes are in place?
Safety netting via ICE system
If the kit is not completed/returned by day 7 after issue, the ICE system will issue a reminder.
On receipt of this reminder, PLEASE DO NOT ISSUE ANOTHER KIT/REQUEST IN ICE.
Please contact the patient and encourage them to complete the test you have already provided.
If the kit is not completed/returned by day 28 after issue, it will be assumed that it is not going to be returned and will be cancelled.
If the kit is completed/returned after 28 days, it will be processed but will be subject to additional administration. In these instances, the results will appear in GP clinical systems as with every other FIT, but it will appear to have been requested by our Consultant Dr Al-Jabouri.
Safety netting in Primary Care - Practices
The CMCA Lower GI suspected cancer pathway guidance includes three possible templates for use in primary care.
- Cheshire and Merseyside safety netting template - developed at Cheshire and Merseyside Cancer Alliance and available to download via CMCA website
- Ardens - available for all practices in C&M within EMIS
- Macmillan safety netting template - available in EMIS as a national template
Practices can choose to use one of these, or have their own system – however it is important that where FIT is not completed/returned, the patient is contacted and advised appropriately.
Will GPs still have to register tests on ICE?
Yes – see ‘FIT GP Information leaflet
What happens if the patient does not complete the test?
See ‘safety netting’ advice above
Can I issue a FIT kit as replacement for a screening programme kit, or use a screening programme kit as a FIT kit ?
No – the kits are processed differently, and the screening programme uses different test parameters. If a patient loses a screening kit, they should request another via the screening channel.
What happens if a patient loses or damages the FIT kit?
If a kit is lost/damaged/contaminated, please start a new request order within ICE. There is no need to cancel the original as the cancellation will not come through to Pathology anyway. Pathology will cancel the previous existing order in the Pathology system upon receipt of the new request.
In these circumstances, please ensure that you get the patient’s commitment to take care to complete the test, to avoid further wastage.
- Useful Information